Abstract
Background and Objectives: Apolipoprotein L1 (APOL1) renal risk variants prevalent in African-ancestry populations are associated with chronic kidney disease (CKD). CKD is a major cause of morbidity and mortality in sickle cell disease (SCD) in adults; yet the prevalence and significance of APOL1 renal-risk variants in this population remains unknown. Our objective was to determine expression of biomarkers of early kidney disease in African American youth with SCD, based on their APOL1 genotype.
Methods:We enrolled65 African American subjects between 5 to 21 years of age with SCD (Hb SS or Hb S β0 thalassemia). Blood and concurrent urine samples were collected. Allenrolled subjects underwent APOL1 genotyping by PCR analysis of DNA extracted from whole blood. Presence of two renal-risk variants qualified for APOL1 High Risk (HR) genotype, while presence of zero or one copy of renal-risk variants qualified for APOL1 Low Risk (LR) genotype.
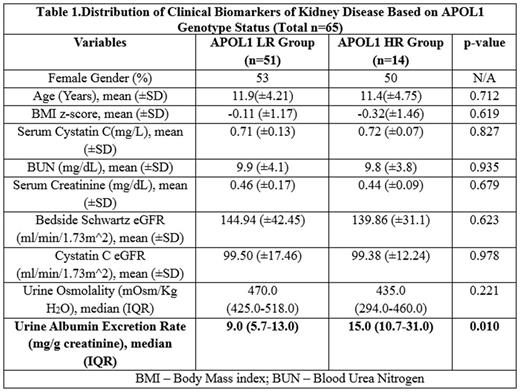
Results: APOL1 HR genotype was expressed by 22% of our subjects; this is comparable to 23% prevalence of APOL1 HR genotype in African American adults with CKD (AASK study). Both groups were similar with respect to distribution of age, BMI z-scores, renal function and urine osmolality. Hyperfiltration was noted in both groups based on low serum creatinine for age, and elevated eGFR based on creatinine clearance. However, median urine albumin excretion rate was significantly higher in HR group vs. LR group.
Conclusions: Preliminary data in this cohort of 65 children and adolescents with SCD detected greater urine albumin excretion rate in the presence of APOL1 HR genotype. Screening SCD youth for heightened CKD risk may be an avenue to initiate targeted preventive interventions to preserve renal function and reduce progression of kidney disease.
Acknowledgment: Research reported in this publication was supported by the National Institute Of General Medical Sciences of the National Institutes of Health under Award Number P20GM109021; National Kidney Foundation Young Investigator Grant and DE-CTR-ACCEL grant number U54-GM104941 (PI: Binder-Macleod).
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal